A potent and selective degrader of the transcription factor STAT3 offers a new approach to a previously “undruggable” target.
12:00 PM
Author |

For decades, a transcription factor known as STAT3 has been a major therapeutic target in the treatment of cancer. But STAT3 has largely been considered "undruggable" due to the difficulty of developing compounds to effectively inhibit its activity.
Researchers at the University of Michigan Rogel Cancer Center have taken a promising new approach to targeting STAT3 — developing a small-molecule compound that harnesses the power of a natural cellular "cleanup" system to get rid of it entirely, rather than traditional methods that have tried to block its action.
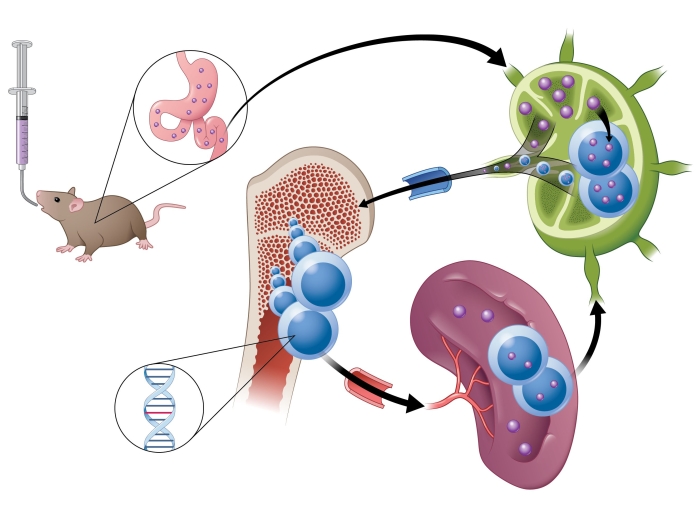
The U-M compound was able to achieve a long-lasting and nearly complete elimination of tumors in mouse models of leukemia and lymphoma, the authors report in Cancer Cell.
In a mouse model of leukemia, the degrader eliminated all of the tumors, with the mice remaining tumor free for 60 days. In two mouse models of lymphoma, the drug eliminated all of the tumors for more than 100 days, when the experiments were ended. The compound was well-tolerated by the mice, the researchers note.
"STAT3 plays a major role in almost every aspect of human cancer — in cancer initiation, progression, metastasis and immune evasion," says study senior author Shaomeng Wang, Ph.D., the Warner-Lambert / Parke-Davis professor and a professor of internal medicine and pharmacology at the U-M Medical School, and a professor of medicinal chemistry at the U-M College of Pharmacy. "It also has been implicated in autoimmune and inflammatory diseases. So, there's a lot of therapeutic potential if one can target STAT3 successfully."
MORE FROM THE LAB: Subscribe to our weekly newsletter
Applying a technology called proteolysis-targeting chimeras (PROTAC), the investigators designed the compound to specifically tag the STAT3 protein for recycling by the body's own cleanup systems. This eliminates the STAT3 protein from cells and tissues and blocks its activity entirely, while leaving the function of related proteins intact.
This new approach circumvents problems seen in drugs aimed at merely inhibiting STAT3's activity, including the accidental targeting of other proteins in the seven-member STAT family, which mediate many aspects of cell growth, differentiation and death, says Wang.
STAT3 plays a major role in almost every aspect of human cancer… So, there's a lot of therapeutic potential if one can target STAT3 successfully.Shaomeng Wang, Ph.D.
The study's co-first authors are Longchuan Bai, Ph.D. and Haibin Zhou, Ph.D., two research scientists in Wang's lab, Renqi Xu, Ph.D., a postdoctoral fellow in Wang's lab, and Yunjun Zhao Ph.D., a former postdoctoral fellow in Wang's lab and currently a professor in Shanghai, China.
The research team, which included collaborators at the U-M Life Sciences Institute's Center for Structural Biology and the College of Pharmacy's Pharmacokinetics and Mass Spectrometry Core, has since further optimized the compound described in the study into a clinical development candidate.
LISTEN UP: Add the new Michigan Medicine News Break to your Alexa-enabled device, or subscribe to our daily updates on iTunes, Google Play and Stitcher.
"In the clinical setting, we hope to be able to achieve a dosing schedule that is convenient for cancer patients, perhaps requiring administration of the drug as little as once a week based upon our study," Wang says.
The group is collaborating with Oncopia Therapeutics, a U-M start-up company, to complete studies required for filing an investigational new drug application with the Federal Food and Drug Administration, which is required to initiate human clinical trials.
The study was funded by a research contract from Oncopia Therapeutics and by the Rogel Cancer Center Core Grant (P30 CA046592) from the National Cancer Institute, part of the National Institutes of Health. The research used resources of the Advanced Photon Source at the Argonne National Laboratory and was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
For disclosure, U-M has filed patent applications on the compound and its analogs. These patents have been licensed by Oncopia Therapeutics, in which Wang is a co-founder, a paid consultant and owns stock. U-M also has a financial interest in Oncopia.
Additional authors on the paper include Krishnapriya Chinnaswamy, Donna McEachern, Jianyong Chen, Chao-Yie Yang, Zhaomin Liu, Mi Wang, Liu Liu, Hui Jiang, Bo Wen, Jennifer L. Meagher, Duxin Sun and Jeanne Stuckey, all of U-M.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!